By Steve Lundeberg
Three years ago a British Columbia man named George Gould developed a bacterial infection following colorectal surgery at a Vancouver hospital.
Antibiotics failed to knock the infection down. Then failed again.
Twenty-two times, Gould was admitted to the hospital for intravenous antibiotics.
Following the last hospital trip, the 58-year-old died, a victim of New Delhi metallo-beta-lactamase, an enzyme behind some so-called “superbugs.”
Superbugs are an evolutionary result of decades of largely unfettered antibiotic use on humans and animals, along with environmental contamination from sources such as antifungals applied to row crops. Also helping to shape the mutations are antimicrobial compounds commonly found in soaps and hand sanitizers.
In November, the Centers for Disease Control and Prevention published its second AR Threats Report, which categorizes as “urgent threats” one fungus, Candida auris, and four bacteria – Carbapenem-resistant Acinetobacter, Clostridioides difficile, Carbapenem-resistant Enterobacteriaceae and drug-resistant Neisseria gonorrhoeae.
In addition, Methicillin-resistant Staphylococcus aureus (MRSA), drug-resistant Streptococcus pneumoniae and drug-resistant tuberculosis are among 11 organisms named as “serious threats.”
A Matter of Time
While new antibiotics have been brought to market recently, few are available as oral options for patients outside of the hospital setting.
“And even with a new antibiotic, it’s only matter of time until resistance arises,” says Jessina McGregor of the College of Pharmacy. “It’s a scary scenario. Consider all of the antibiotics that are given prophylactically for surgeries to head off surgical site infections. Antibiotic resistance may progress to the point where surgeries that are now considered safe could potentially be very risky because of the threat of infection.”
Such as Caesarean sections, for example, which account for one-third of all U.S. births. Or the 700,000 knee replacements that currently happen every year. Or the 400,000 hip replacements.
Slowing down the march of drug resistance depends in large part on using antibiotics as responsibly as possible – practicing what scientists like McGregor call “antibiotic stewardship.”
A big part of that, of course, falls on the providers who have the dual mission of optimizing patient outcomes while also restraining the pace at which bacteria mutate to fend off drugs previously used to kill them.
Ask Your Doctor
But patients should take an active role as well, by asking questions of their health-care providers. Questions such as, what diseases is the suggested antibiotic effective against? How sure are you that I have one of those? Are there other drug options, and what are the pros and cons of each? What are the potential side effects?
And perhaps most importantly, is an antibiotic even needed?
“Among the general public, I think there’s a lot of misconception about what antibiotic resistance is,” says McGregor, who researches ways to measure and inform antibiotic stewardship. “There isn’t widespread understanding that we even have different antibiotics, and what the differences are between them, or that when a doctor selects an antibiotic, the decision can have an impact both on the individual patient and the entire community of current and future patients.”

Some providers tend to hedge their bets by choosing an aggressive antibiotic like ampicillin or amoxicillin that will work on a wide variety of pathogens, which sounds good – except that it also gives lots of organisms a chance to develop resistance against the drug.
Alternatively, a provider can take a more conservative approach, picking an antibiotic such as erythromycin or vancomycin that’s effective against a smaller range of bacteria. That choice provides more protection against resistance but might not be effective on the patient’s illness if the wrong narrow-spectrum drug is chosen.
“Infectious diseases and antibiotic use are a bit different than other medication utilization areas, because you’re treating a condition that’s caused by another living organism, something that can change in response to the treatment you’re giving.” McGregor says.
Resistance Predicted
Alexander Fleming, a Scottish physician and researcher, stumbled upon the first modern antibiotic in 1928 when he noticed mold spores that had gotten into a Petri dish were killing staphylococcal bacteria.
Seventeen years later, when penicillin had become a mass-produced pharmaceutical, Fleming included a warning in his Nobel Prize acceptance speech: “It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body…. There is the danger that the ignorant man may easily underdose himself, and by exposing his microbes to non-lethal quantities of the drug, make them resistant.”
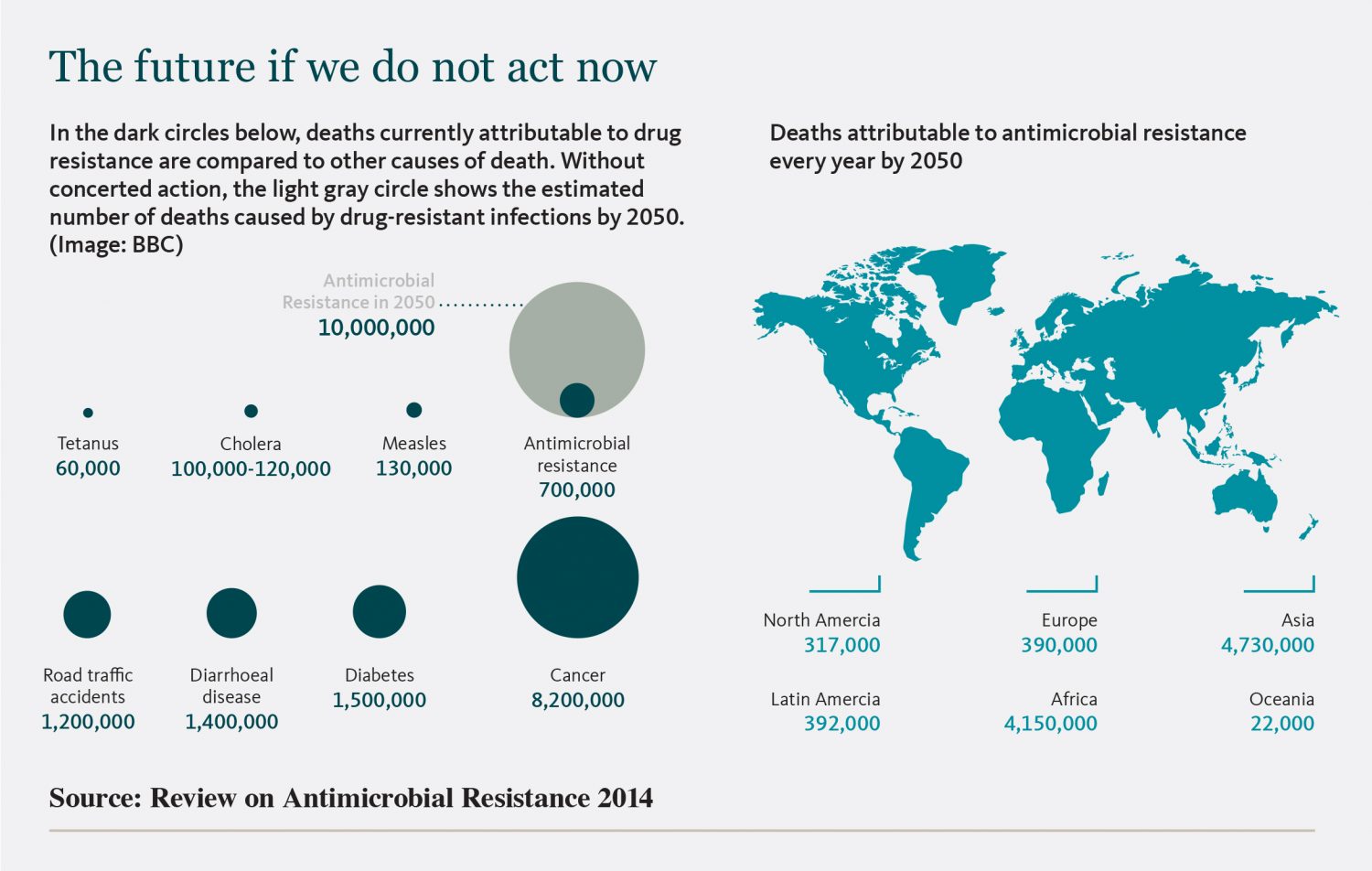
Fleming, as the latest CDC report indicates, could hardly have been more spot-on in his prediction. Not quite a century after his discovery, antibiotic-resistant bacteria now sicken nearly 3 million people in the United States every year and kill more than 35,000.
That means someone develops an antibiotic-resistant infection every 11 seconds, and someone dies from one of those infections every 15 minutes.
When the CDC did its first AR Threats Report six years ago, the numbers were 2 million sickened and 23,000 fatalities. Neither report includes viruses or parasites but both include fungal infections.
The CDC report noted that hospitals have improved their methods for tracking and slowing the spread of resistant organisms, and that hospital deaths from superbug infections have decreased by nearly 30% since 2013.
The report also cited, however, rising numbers of resistant infections outside of hospitals, an important target area for improved antibiotic stewardship.
In a study, published in December 2019 in BMJ, McGregor and scientists from Oregon Health & Science University, Portland State University and Pacific University found that antibiotic prescriptions written for “ambulatory care” patients in the U.S. – i.e., those not admitted to a hospital – lacked a documented reason 18% of the time.
“Prescribing without documenting the indication likely means there’s a significant underestimation of the scope of the problem of unnecessary antibiotic prescribing,” McGregor says. “In some care settings, providers have worked hard to improve stewardship, yet in other settings more evidence is needed to direct providers to the best strategies for antibiotic use.”
Dental Practice
Such as the dentist’s office, given that 10% of all antibiotic prescriptions in the United States are written by dentists.
In another 2019 paper, published in May in JAMA Network Open, McGregor and collaborators from the University of Illinois at Chicago and Northwestern University used a national health care claims database to examine 170,000 dentist-written antibiotic prescriptions
They learned that, based on medical guidelines, those prescriptions were unnecessary at the rate of 81%.
“As technology has changed, as health care records have become more electronic, we have a new capacity to study how well we’re using antibiotics, and the impact of our actions on patients and communities,” McGregor says. “We have a new capacity to implement prudent use of antibiotics, plus we’re better able to inform how we define prudent use.”
She emphasizes that it’s important for every patient to understand that getting an aggressive antibiotic today may impact whether that same antibiotic will work for you in the future, or your spouse or child.
“People need to take more ownership so they understand the medicines they’re taking and why,” McGregor says. “It’s good to ask questions of your provider and understand what alternatives there might be, and it’s good for both sides to engage in a dialogue to understand what the pluses and minuses are for different treatments.”




