By Jens Odegaard
Editor’s Note: This story stems from “Engineering Out Loud,” a podcast produced by the OSU College of Engineering. You can listen to this episode online. Starting its ninth season in the fall of 2019, “Engineering Out Loud” tackles emerging issues from health care to the environment. Subscribe to the podcast on Apple Podcasts, Spotify or Stitcher.
How can we help in the fight against Parkinson’s disease? Harriet Nembhard and her colleagues developed a sensor system to detect the disease early on, opening the door to earlier treatment and improved quality of life. Nembhard is the head of the School of Mechanical, Industrial and Manufacturing Engineering and Eric R. Smith Professor of Engineering at Oregon State University.
“So slight and nearly imperceptible are the first inroads of this malady, and so extremely slow is its progress, that it rarely happens that the patient can form any recollection of the precise period of its commencement. The first symptoms perceived are, a slight sense of weakness, with a proneness to trembling in some particular part; sometimes in the head, but most commonly in one of the hands and arms. These symptoms gradually increase in the part first affected; and at an uncertain period, but seldom in less than 12 months or more, the morbid influence is felt in some other part.”
JENS ODEGAARD: That was an excerpt from an 1817 essay describing a disease known as “The Shaking Palsy.” It was written by a British doctor. You might have heard of him. Dr. James Parkinson. This season on “Engineering Out Loud,” we’re sharing stories of how our researchers are helping us stay healthy and safe. Today’s show is about work being done by an industrial engineer and her team to assist the early detection of Parkinson’s disease, or as it’s sometimes called, PD.
HARRIET NEMBHARD: One of the things that I often start with is to say, “By show of hands, how many of you know somebody with Parkinson’s disease?” And often, a quarter to a third of the hands go up in the room. PD affects 2% of all seniors, so its prevalence in the U.S. is about 1 million people, and 60,000 cases are diagnosed each year.
ODEGAARD: Overall, Parkinson’s disease affects more than 10 million people worldwide. And the symptoms are the same that Dr. James Parkinson documented more than 200 years ago. Though today, we know a bit more about what’s actually going on inside our brains, particularly in something called the substantia nigra, a structure in the midbrain.

NEMBHARD: Parkinson’s disease is a neurodegenerative disorder. And what we do know from neuroscience is that the nerve cells in the substantia nigra produce the dopamine.
ODEGAARD: This dopamine is released onto motor control areas of the brain to facilitate smooth, coordinated movements of the body’s muscles.
NEMBHARD: And in Parkinson’s disease, it’s those nerve cells that are dying. As those nerve cells die, then one side of the substantia nigra perhaps becomes compromised first, and then that is what results in the asymmetry of the movement.
ODEGAARD: What normally comes to mind when we think of the symptoms of Parkinson’s disease are tremors and slow movements. But early on there are signs of asymmetrical movement. Normally, our limbs work in coordination with each other. Our arms swing back and forth as we walk. But when Parkinson’s sets in, those kinds of movements can become less coordinated. It’s this asymmetrical movement that could help in early detection of the disease.
NEMBHARD: So for me as an industrial engineer, I see that diagnosis of those 60,000 cases as a system, a PD detection system. And right now I would say that system has a few cracks in it because often patients are diagnosed much later in the development of the disease and are unable to get early treatment. It’s not a curable condition. So early detection and treatment is really the key.
ODEGAARD: Nembhard and her colleague Conrad Tucker, an associate professor of engineering at Penn State University, brought together a team of people from a variety of backgrounds to look into the issue. They were leading the National Science Foundation Center for Health Organization Transformation and tapped into the talents of researchers from engineering, health policy, information sciences and medicine.
NEMBHARD: We were working with a neurologist who expressed some frustration in some sense about the fact that by the time patients came to her, it was already pretty clear that they had had Parkinson’s disease for some time.
ODEGAARD: That neurologist was Xuemei Huang, also from Penn State. They asked Xuemei, “How could you possibly know this?” It turns out that somebody with her specific training can recognize certain movements of a patient in the very early stages of Parkinson’s disease. And she didn’t even have to be in the same room to recognize the symptoms.
NEMBHARD: This same colleague had taken a look at some 20 years of footage of Michael J. Fox. There was one scene in which there is an explosion that happens behind him, and he turns around at the noise. But you could see very clearly that one arm extended out in that turn and the other arm kind of stayed by the side. For a neurologist, that asymmetrical movement is an important early tell.
ODEGAARD: Michael J. Fox is an actor famous for playing Marty McFly in the “Back to the Future” trilogy. His Parkinson’s disease was diagnosed in 1991 when he was only 29 years old. But the symptoms Xuemei had observed were from even earlier performances. His diagnosis was devastating. Here’s how he described it in a 2017 interview on “CBS Sunday Morning.”
ODEGAARD: Although early diagnosis is heartbreaking, it’s important because starting treatment can help manage the symptoms, which get worse with time.
NEMBHARD: What typically happens, if you can treat it early, is that you can start Levodopa-based medications, which help the body to produce dopamine, and compensate for those low dosages of dopamine. So, getting that treatment early is really one of the main ways we have to combat it. And if we don’t start it until later, it’s harder to really improve quality of life for the patients.
ODEGAARD: Many cases of Parkinson’s are missed or misdiagnosed in the early stages. So Harriet and her team were driven to develop a system that could detect asymmetrical movement early on.
NEMBHARD: And we started doing some digging into that and thinking about it as, “Well, how can we perhaps translate the ability of that neurologist to see these physical manifestations into a system?”
ODEGAARD: The team had a few design goals in mind. One obviously was detecting Parkinson’s early. They also wanted a scalable system that they could get into the hands of as many people as possible. Finally, they wanted sensors that weren’t intrusive or intimidating.

to a person to detect the likelihood of
asymmetrical movement. (Illustration: Heather Miller)
NEMBHARD: There’s a software developer kit that you can use to access control of the cameras and the sensors. And rather than connecting it to a game console, we connected it to a computer just to collect the data that we needed.
They decided to borrow from video gamers. Seriously! They used the Microsoft Kinect sensor from the Xbox 360. You remember. Using your body as the controller to knock out characters with your avatar, racing cars and scoring the game-winning free kick. Though the technology now is pretty dated for video games, it was perfect for what they were trying to accomplish.
ODEGAARD: The way the system works in detecting asymmetrical movement is fairly straightforward.
NEMBHARD: All we really need is for the patient to walk toward the Kinect sensor. The first simple test is walk forward, walk back, walk to the left, walk to the right, within a marked-off box.
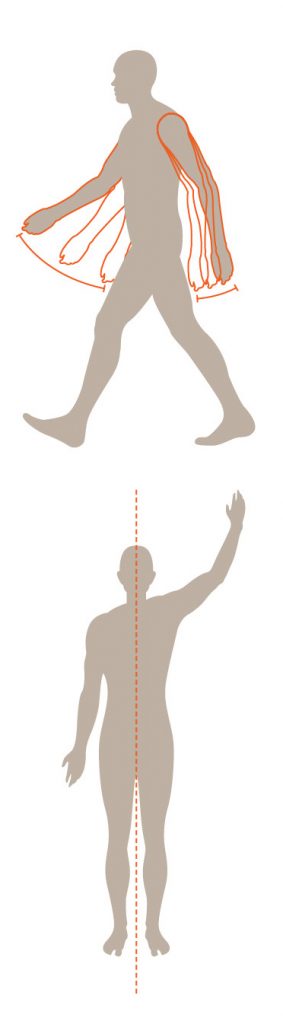
the left and right, sensors gather data on
patterns of movement. (Illustration: Heather Miller)
ODEGAARD: Harriet and her team programmed the sensor to collect positional data from 20 different areas on the patient’s body. These areas included the head and shoulders, chest, elbows, wrists, knees and ankles. Data are collected at a rate of about 10 observations per second at each area. From there, it’s on to some computational number crunching.
NEMBHARD: Then the data analysis phase is really about looking at all of those observations using some machine-learning algorithms. We wrote several machine-learning algorithms to go through that data and flag for each node what is symmetrical movement or not. And so it’s a very specific spatial and mathematical comparison on a node-by-node basis to say: “Yes, there’s PD risk in this pair of movements,” or “No, there is not.”
ODEGAARD: These algorithms work in such a way that detection of asymmetrical movement is tailored to the individual patient.
In theory, the detection system could be used to assist a health care provider in diagnosing Parkinson’s by providing fast, automated detection of some of the earliest signs of the disease. It could also be used in ongoing treatment of those with Parkinson’s by helping detect if medications are effectively treating the symptoms.
Harriet and her team have shown the idea works. But before something like it shows up in your doctor’s office or community care clinic, there are still a lot of questions to answer about how it would be implemented in the health care process.
As an educator, Harriet is using the research as a foundation for
her students to build upon and attempt to answer some of
these questions.

One group of undergraduates experimented with the Kinect software developer kit to help tailor the system’s functionality.
NEMBHARD: I had another group — it was a mechanical engineering student and two industrial engineering students — to help design a portable, adjustable, safe console for such a system. If you’re imagining that we’re taking it to different community health fair settings, it’s not just like we can take it out of our case and put it on a counter, right? How can you really make it portable? So they looked at that.
ODEGAARD: She had some more industrial engineering students imagine a completely different scenario — this one for reaching people in remote locations or those who have difficulty leaving the house.
NEMBHARD: You know, how many seniors are in Oregon? What if we wanted to do this in a telehealth capacity? What would potentially be the cost? What would be the potential hurdles? So it’s been exciting to see it, become this teaching tool.
ODEGAARD: This whole project inspired Harriet to think about other ways a collaborative team could improve human health.
NEMBHARD: And so, one of the questions that I’ve been interested in is: Could using sensors and machine learning be helpful in the detection and management of concussions?
ODEGAARD: She doesn’t have an answer yet. But she imagines a potential system that would include coaches, team doctors, other players and potentially even some type of monitoring system based on game footage. This system would help detect concussions and help in the support and recovery from those injuries.
NEMBHARD: Well, I will say, as an academic leader, I’m particularly concerned with the impact of concussions and CTE on football student-
athletes’ health.
ODEGAARD: CTE stands for chronic traumatic encephalopathy, which is a condition associated with both concussions and repeated blows to the head.
NEMBHARD: So this is well understood that football puts people at higher risk of concussion and potentially for a future onset of chronic traumatic encephalopathy, or CTE. So I think that all of that becomes a part of a system, but those are some of the questions that certainly interest me. How can we really do all that we can do, while these players are in our charge, to make sure that we protect their health and safety?
ODEGAARD: Harriet is in the early stages of pulling together an interdisciplinary team of people from across Oregon State and beyond to start addressing this medical issue.
ODEGAARD: In reckoning with his own diagnosis, Michael J. Fox decided to go all in on scientific research and development. So he founded the Michael J. Fox Foundation for Parkinson’s Research. He had this to say about the importance of medical research:
This progress all starts with people like Harriet, who take action
and use their skills to support the health and success of those
around them.
NEMBHARD: I love everybody who’s here. I’m excited about investing in their success, right? I’m excited about making sure that we have an academic community where we have each other’s backs, that we’re encouraging each other to really thrive.





